转自:康龙化成
AGeneralPlatformforCu(II)-CatalyzedHydrofunctionalizationofUnactivatedAlkynesviaπ-LewisAcidActivation
JuntaoSun†,ThomasH.Tugwell‡,MithunC.Madhusudhanan‡,LetianXu†,ShenghuaYang†,PengLiu‡*,andKearyM.Engle†*
†DepartmentofChemistry,TheScrippsResearchInstitute,10550NorthTorreyPinesRoad,LaJolla,California,92037,UnitedStates;‡DepartmentofChemistry,UniversityofPittsburgh,219ParkmanAvenue,Pittsburgh,Pennsylvania,15260,UnitedStates
—ChemRxiv,2025,10.26434/chemrxiv-2025-pmqhx
RecommendedbyRuiJin_MC5

KEYWORDS:Cucatalysis,stereoselective;hydrofunctionalization(反应类型),C(sp2)-H,C(sp2)-O,C(sp2)-N(成键类型),alkynes,carboxylicacids,acidicOH-nucleophiles,sulfonamides(原料),trisubstitutedalkenes,enolether,enamine,alkylideneβ-lactam(产物),π-Lewisacidactivation(其他)
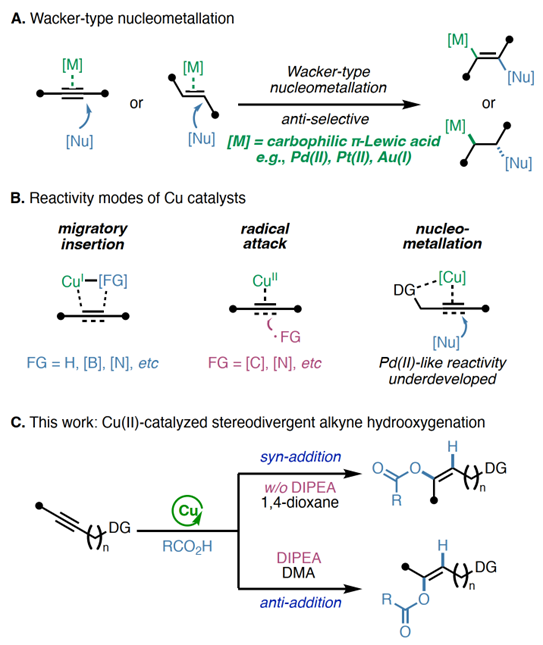
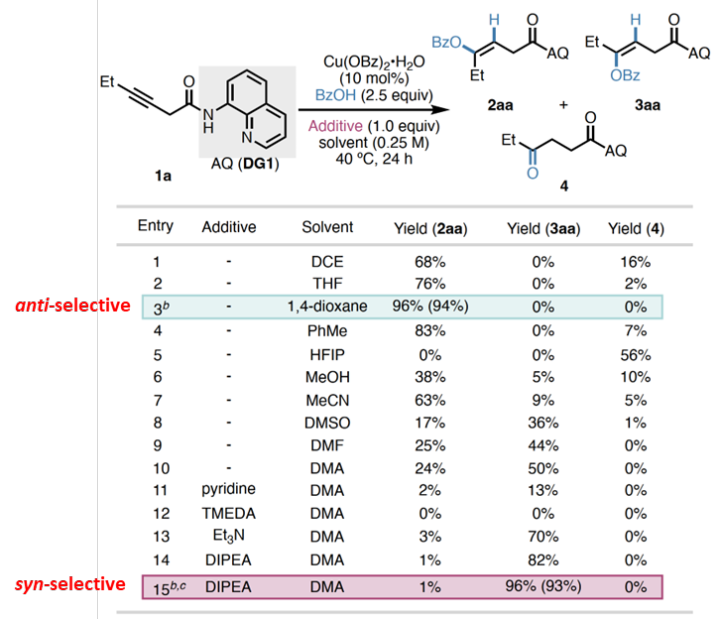
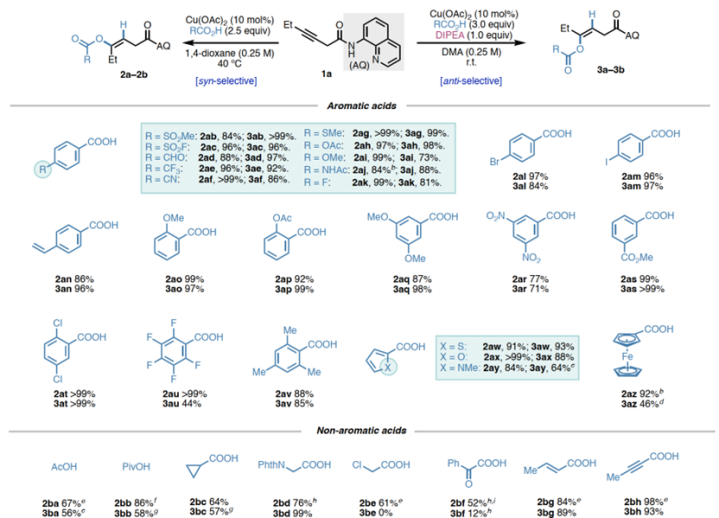
ABSTRACT:Acopper(II)-catalyzedstereodivergenthydrooxygenationofelectronicallyunactivatedalkyneswithcarboxylicacidsisreported.Regioselectivityandkineticreactivityarefacilitatedbyabidentateauxiliary,andsyn-oranti-stereoselectivityiscontrolledthroughjudicialtuningofreactionconditions.ThemethodaffordstrisubstitutedE-orZ-alkeneswithenolesterfunctionalityinahighlyselectivemanner.Beyondcarboxylicacids,avarietyofotherOH-andNH-nucleophilesreactsmoothlytofurnishenolether,enamineandalkylideneβ-lactambuildingblocks.Mechanisticexperimentsanddensityfunctionaltheory(DFT)calculationsshedlightonthenatureofπ-LewisacidactivationwithCu(II)andsupportacatalyticcyclethatfeaturesinner-spherenucleocuprationmechanismvia6-memberedtransitionstateforsyn-additionandbase-assistedouter-spherenucleocuprationmechanismforanti-addition.
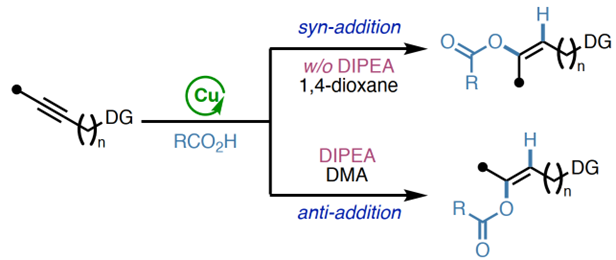
Backgroundandthiswork
Conditionscreening
Substratescopeofcarboxylicacids
Substratescopeofalkynes
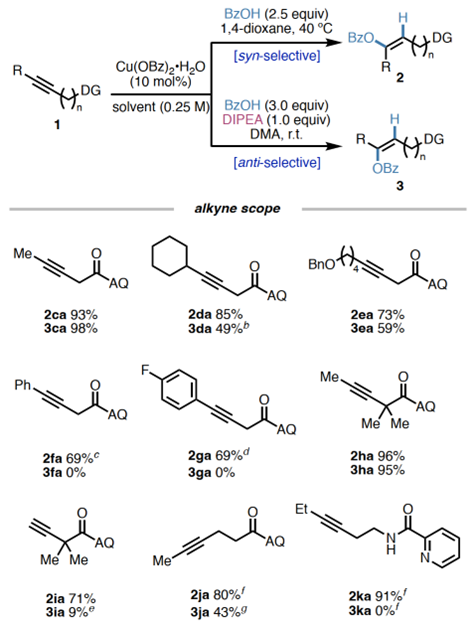
Substratescope:OH-andNH-nucleophiles
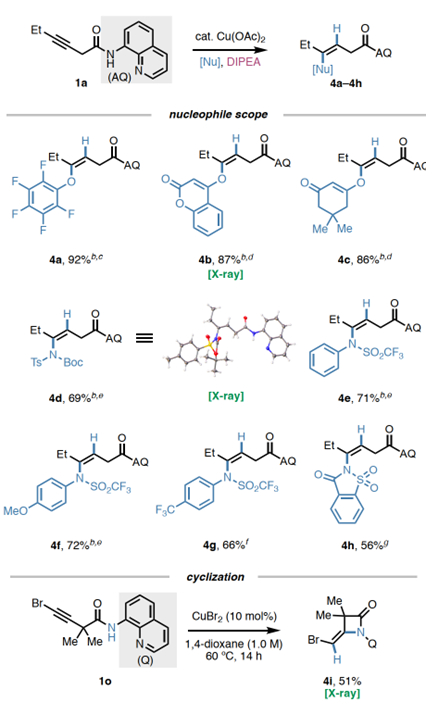
Applications:Gram-scaleexperimentsandproducttransformations
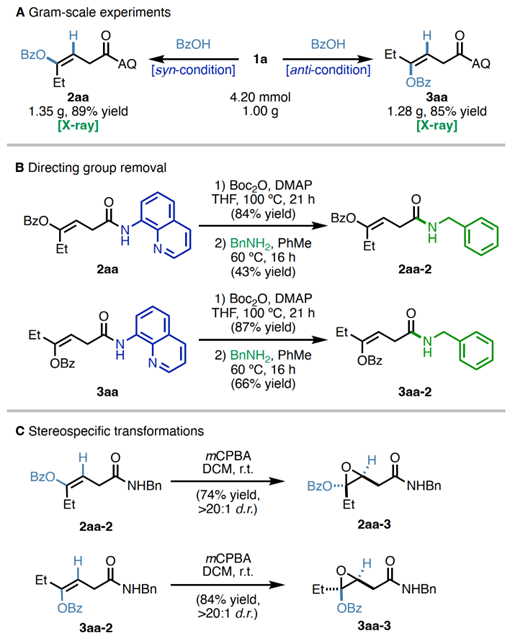
Proposedmechanism
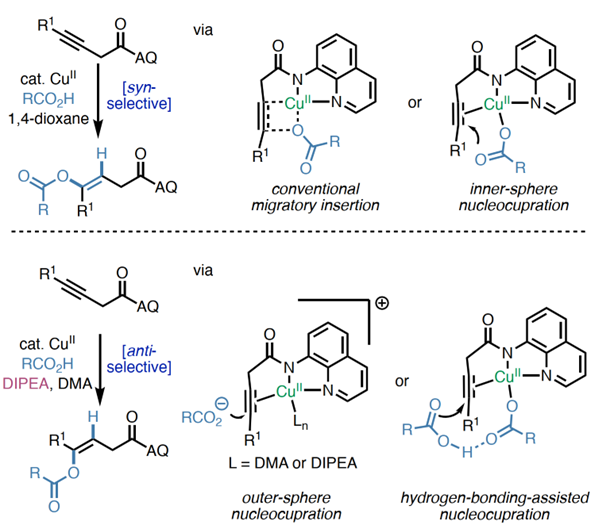
Inconclusion,Prof.KearyM.EngleandProf.PengLiuetaldevelopaversatileplatformemployingCu(II)asπ-LewisacidtoachieveofelectronicallyunactivatedalkyneswithdiverseOH-andNH-nucleophiles.Regioselectivityandkineticreactivityarefacilitatedbyabidentateauxiliary,andsyn-oranti-stereoselectivityofbenzoicacidcouplingpartnersiscontrolledthroughjudicioustuningofreactionconditions.Analkynesubstratebearingamono-dentateamidealsoexhibitsreactivityunderamoreforcingcondition.MechanisticexperimentsandDFTcalculationssupportaninner-spherenucleometallationmechanismviaa6-memberedtransitionstateinsyn-additionandhighlighttheessentialrolesofDIPEAandDMAintheanti-additionpathway.WeanticipatethatthisCu(II)-catalyzedπ-activationstrategycangeneralizedtootherπ-bondcontainingsubstrates,providingasustainablealternativetopreciousmetalcatalysts.
Scripps研究所Engle课题组和匹兹堡大学刘鹏课题组共同开发了一个以Cu(II)作为π-路易斯酸的多功能催化平台,成功实现了非活化炔烃与各类OH/NH亲核试剂的区域选择性和立体选择性偶联。通过双齿导向基团的辅助作用实现区域选择性和动力学控制,并借助反应条件的精准调控分别获得苯甲酸偶联的顺式或反式立体选择性产物。研究还发现,含单齿酰胺基团的炔烃底物在更剧烈条件下同样表现出反应活性。机理实验与DFT计算共同证实:顺式加成通过六元环过渡态的内球型金属亲核机制进行,而反式加成路径中DIPEA和DMA溶剂起着关键作用。预期这种Cu(II)催化的π-键活化策略可拓展至其他含π-键底物,为贵金属催化剂提供可持续的替代方案。